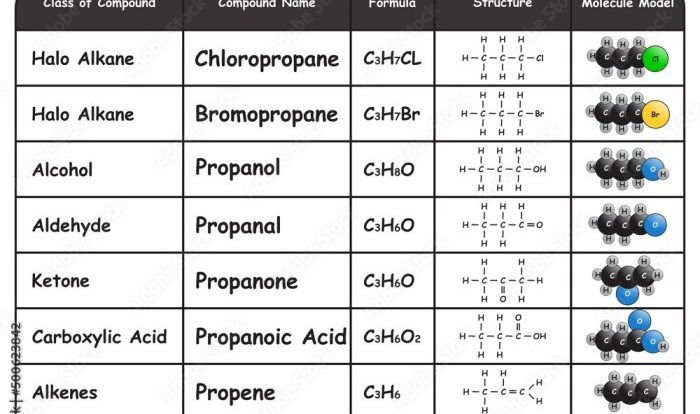
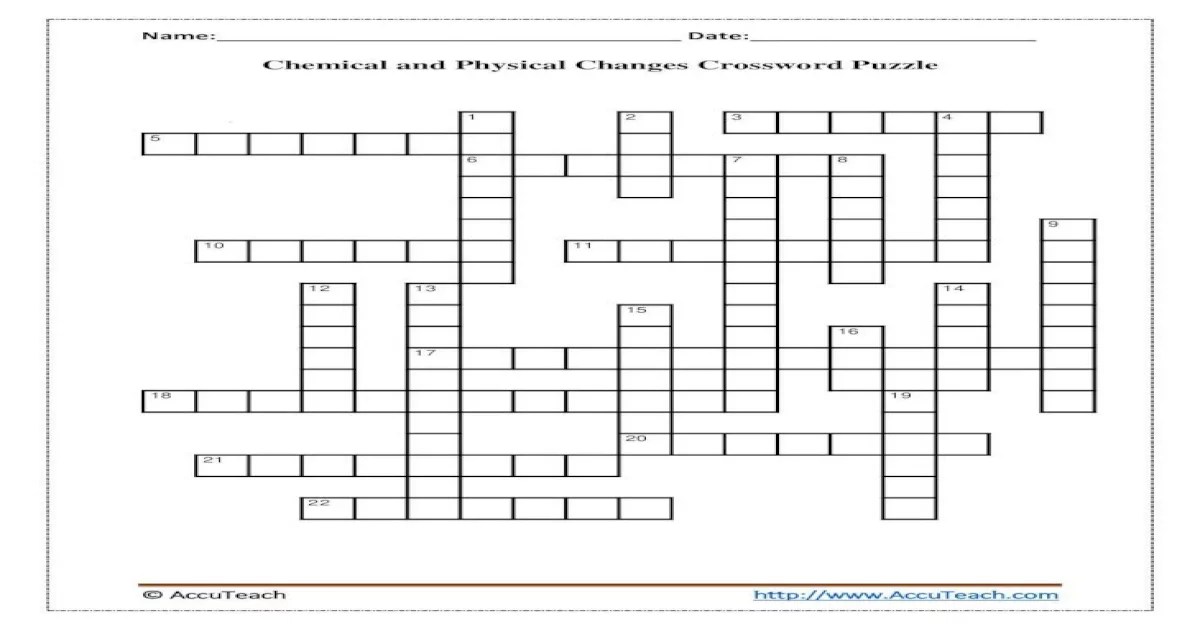
Embark on a captivating journey with the Physical and Chemical Changes Crossword Puzzle, where you’ll unravel the secrets of matter’s transformations. This engaging puzzle invites you to explore the fundamental concepts of physical and chemical changes, unraveling the mysteries of how substances can alter their states and compositions.
Delve into the realm of physical changes, where substances undergo reversible transformations without altering their chemical identities. Witness the melting of ice, the freezing of water, and the sublimation of dry ice. Then, venture into the fascinating world of chemical changes, where atoms rearrange to form entirely new substances, exemplified by the burning of wood, the rusting of iron, and the baking of bread.
Physical and Chemical Changes: Physical And Chemical Changes Crossword Puzzle
Physical changes are changes in the form or appearance of a substance without altering its chemical composition. They involve changes in the physical state of a substance, such as melting, freezing, and sublimation. Physical changes are reversible, meaning that the original substance can be recovered by reversing the change.Chemical
changes, on the other hand, involve the rearrangement of atoms to form new substances. They involve a change in the chemical composition of the substance and are irreversible. Examples of chemical changes include burning, rusting, and baking.
Physical Changes
Physical changes are changes in the form or appearance of a substance without altering its chemical composition. They involve changes in the physical state of a substance, such as melting, freezing, and sublimation.
- Meltingis the change of a solid to a liquid.
- Freezingis the change of a liquid to a solid.
- Sublimationis the change of a solid directly to a gas.
Physical changes are reversible, meaning that the original substance can be recovered by reversing the change. For example, water can be melted into a liquid and then frozen back into a solid.
Chemical Changes, Physical and chemical changes crossword puzzle
Chemical changes involve the rearrangement of atoms to form new substances. They involve a change in the chemical composition of the substance and are irreversible. Examples of chemical changes include burning, rusting, and baking.
- Burningis a chemical reaction that involves the combination of a substance with oxygen, releasing heat and light.
- Rustingis a chemical reaction that occurs when iron is exposed to oxygen and water, forming iron oxide.
- Bakingis a chemical reaction that involves the combination of ingredients to form a new substance, such as bread.
Chemical changes are irreversible, meaning that the original substance cannot be recovered by reversing the change. For example, once wood is burned, it cannot be converted back to its original form.
Essential FAQs
What is the difference between a physical change and a chemical change?
A physical change alters a substance’s form or appearance without changing its chemical composition, while a chemical change involves the rearrangement of atoms to form new substances with different properties.
Can physical changes be reversed?
Yes, physical changes are generally reversible, meaning the substance can return to its original form under certain conditions.
How can I identify a chemical change?
Chemical changes are often accompanied by the release or absorption of energy, the formation of gas, or a change in color.