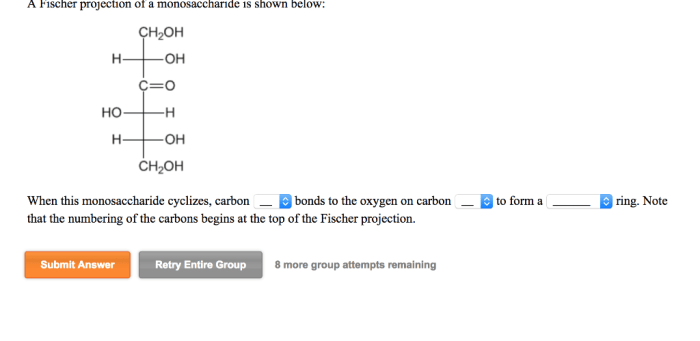
A Fischer projection of a monosaccharide is shown below, providing a simplified representation of its three-dimensional structure. Fischer projections are invaluable tools in carbohydrate chemistry, enabling the visualization and understanding of the stereochemistry and properties of these essential biomolecules.
This Artikel explores the significance of Fischer projections in deciphering the intricate world of monosaccharides, guiding readers through their interpretation and applications in various scientific disciplines.
Introduction to Fischer Projections
Fischer projections are two-dimensional representations of monosaccharides, a class of carbohydrates. They depict the three-dimensional structure of monosaccharides in a simplified and standardized manner, providing a valuable tool for understanding their stereochemistry and properties.
Fischer projections are named after Emil Fischer, a German chemist who developed them in the late 19th century. They are widely used in carbohydrate chemistry, biochemistry, and organic chemistry to represent the structure and properties of monosaccharides.
Stereochemistry of Monosaccharides
Chirality of Monosaccharides
Monosaccharides are chiral molecules, meaning they exist in two non-superimposable mirror-image forms called enantiomers. The chirality arises from the presence of one or more chiral centers, which are carbon atoms bonded to four different groups.
D and L Configurations, A fischer projection of a monosaccharide is shown below
Fischer projections assign the D or L configuration to monosaccharides based on the orientation of the hydroxyl group on the chiral carbon furthest from the carbonyl group. If the hydroxyl group is on the right in the Fischer projection, it is a D-monosaccharide; if it is on the left, it is an L-monosaccharide.
Representing 3D Structure
Fischer projections represent the three-dimensional structure of monosaccharides by projecting the molecule onto a plane. The vertical lines represent bonds extending out of the plane, while the horizontal lines represent bonds lying in the plane. The chiral centers are represented by intersections of lines, and the hydroxyl groups are indicated by small circles attached to the lines.
Interpreting Fischer Projections
To interpret Fischer projections, follow these guidelines:
- Vertical lines represent bonds extending forward from the chiral center.
- Horizontal lines represent bonds extending backward from the chiral center.
- Chiral centers are represented by intersections of lines.
- Hydroxyl groups are represented by small circles attached to the lines.
- The D or L configuration is determined by the orientation of the hydroxyl group on the chiral carbon furthest from the carbonyl group.
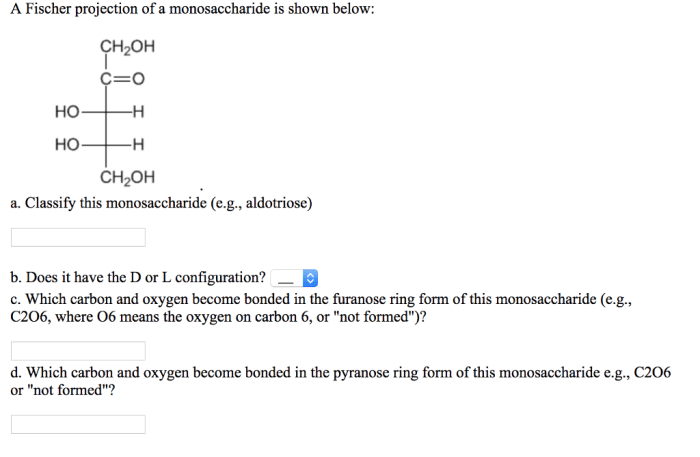
Properties of Monosaccharides from Fischer Projections: A Fischer Projection Of A Monosaccharide Is Shown Below

Fischer projections can reveal various properties of monosaccharides, including:
- Molecular formula:The number of carbon, hydrogen, and oxygen atoms can be determined by counting the atoms in the Fischer projection.
- Number of chiral centers:The number of chiral centers is equal to the number of intersections of lines in the Fischer projection.
- Relative configuration of hydroxyl groups:The Fischer projection shows the relative positions of the hydroxyl groups on the chiral centers.
- Optical activity:The D or L configuration determines whether the monosaccharide is dextrorotatory (+) or levorotatory (-).
Examples of Fischer Projections
Here are some examples of Fischer projections for common monosaccharides:
| Monosaccharide Name | Fischer Projection | Molecular Formula | Number of Chiral Centers |
|---|---|---|---|
| D-Glucose |
H | C=O | / \ C C / \ / \ H C H C H | | | | OH OH OH OH |
C6H 12O 6 | 4 |
| D-Fructose |
H | C=O | / \ C C / \ / \ H C C H | | | | OH OH OH OH |
C6H 12O 6 | 3 |
| D-Galactose |
H | C=O | / \ C C / \ / \ H C H C H | | | | OH OH OH OH |
C6H 12O 6 | 4 |
Applications of Fischer Projections
Fischer projections have wide applications in various fields:
- Carbohydrate chemistry:Fischer projections are used to study the structure, reactivity, and synthesis of carbohydrates.
- Biochemistry:Fischer projections are essential for understanding the structure and function of carbohydrates in biological systems.
- Organic chemistry:Fischer projections are used to represent the stereochemistry of organic molecules, particularly carbohydrates and other chiral compounds.
Question & Answer Hub
What information can be derived from a Fischer projection?
Fischer projections provide information about the molecular formula, number of chiral centers, relative configuration of hydroxyl groups, and optical activity of a monosaccharide.
How do Fischer projections represent the three-dimensional structure of monosaccharides?
Fischer projections represent the three-dimensional structure of monosaccharides by projecting the molecule onto a plane, with horizontal lines representing bonds pointing towards the viewer and vertical lines representing bonds pointing away from the viewer.